 Products with confirmed formulation properties and safety
Products with confirmed formulation properties and safety
We provide complete support from product planning based on market analysis and academic research to notification. Our rich history in this vertical has positioned us as Japan’s leader of functional food claim notifications. We have a large lineup of products whose formulation suitability and stability has already been approved thus allowing us to fast-track the notifications process.
 Products with confirmed formulation properties and safety
Products with confirmed formulation properties and safety
 As of April, 2024
As of April, 2024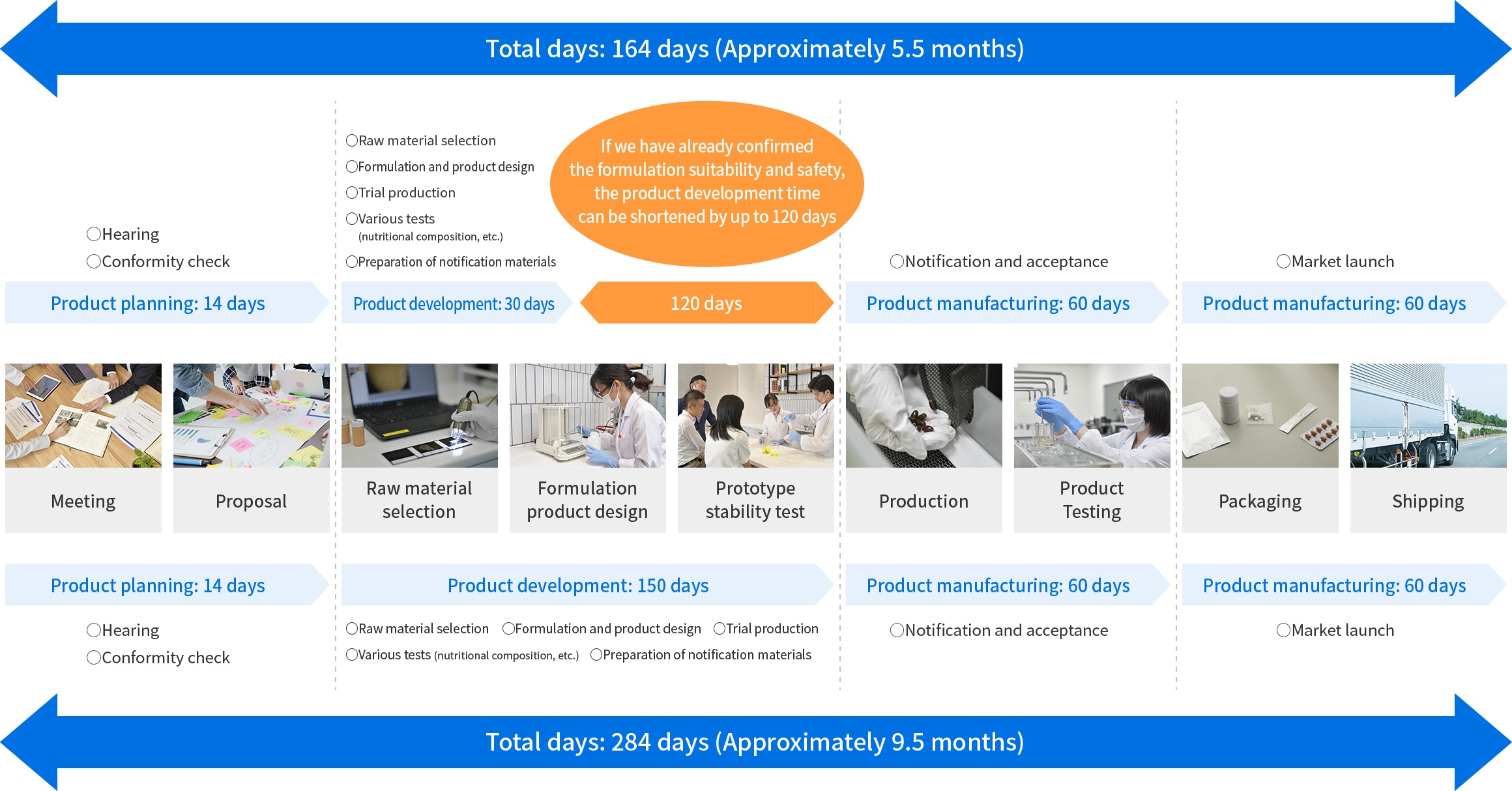

To ensure high quality, all processes from receipt of raw materials and packaging materials to shipment must fulfill our stringent standards throughout the entirety of the manufacturing process. Our equipment and machinery are carefully maintained, and our personnel are knowledgeable and well-trained on the latest techniques.
Sunsho Pharmaceutical has meticulously constructed a system to maintain excellent quality based on the GMP and HACCP tenets. As part of our continued commitment to traceability, we also ensure that production records are kept for a minimum of five years and that all manufactured lot samples are kept for a minimum of three years.


Sunsho Pharmaceutical proposes the most suitable packaging solution for your products based on a number of perspectives, consisting of stability, ease of ingestion, UV protection, and hygienic considerations.
We can provide packaging services as a standalone project or offer our fully integrated support from product development and manufacturing to packaging. Please consult with us to discover which options are relevant to you.







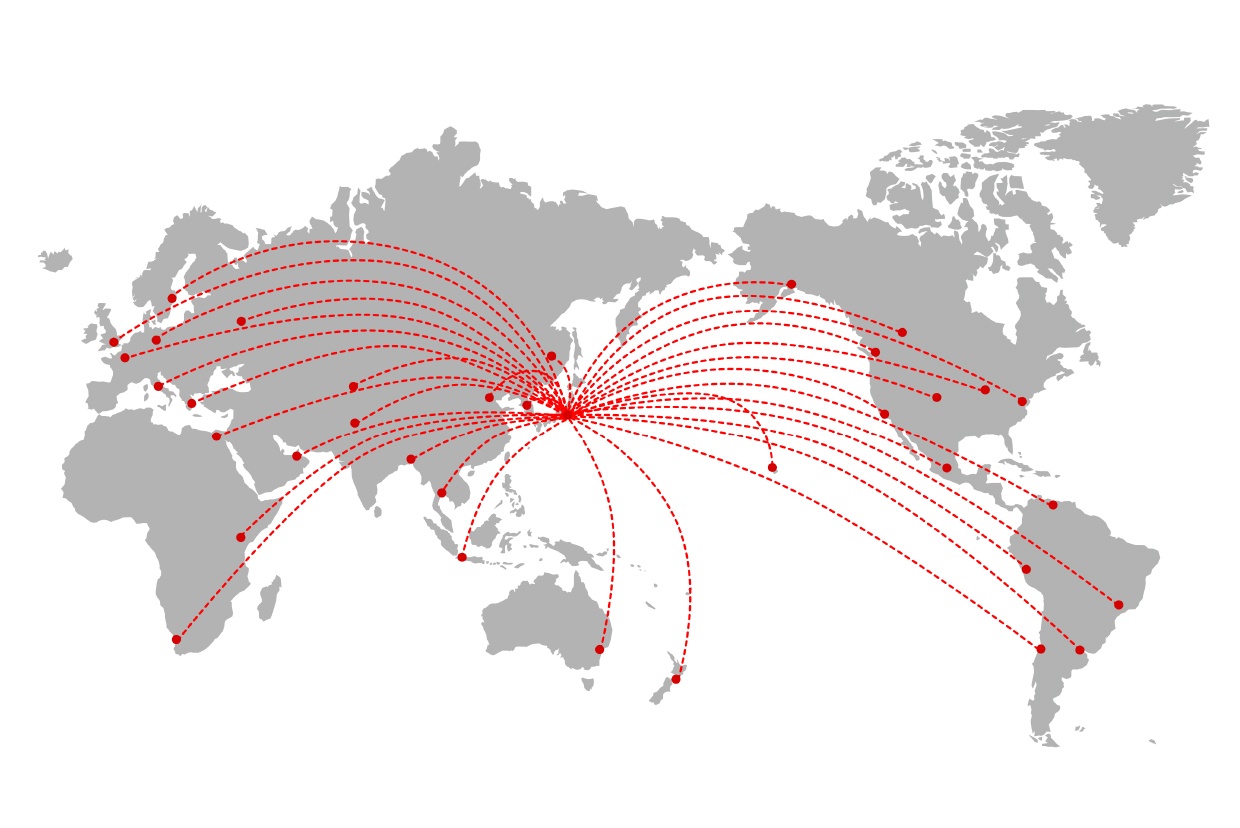
With more than 200 exports a year and our expertise, we can offer a solid proposal to clients that are considering international expansion. Additionally, we also can handle the regulatory requirements of major exporting countries and assist our customers with the documentation for international product registration.
Customers can trust us to handle the exportation processes quickly and efficiently.
